Delray Medical Center becomes first hospital in Florida to treat Alzheimer's patient with non-invasive focused ultrasound technology
Delray Medical Center recently became the first hospital in Florida to treat an Alzheimer’s disease patient using non-invasive focused ultrasound technology as part of a groundbreaking study being conducted in collaboration with Florida Atlantic University’s (FAU) Institute for Human Health and Disease Intervention.
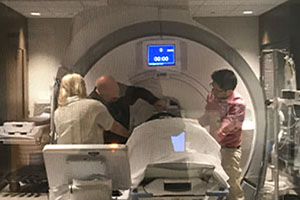 In the FDA-approved clinical trial, focused ultrasound technology is used to disrupt the blood-brain barrier in participating Alzheimer’s patients. Alzheimer’s may be caused by a buildup of certain proteins in the brain. Precisely guided by magnetic-resonance imaging, ultrasound waves are directed at specific areas of the patient’s brain to create a temporary opening in the blood-brain barrier where the protein buildup may be reduced. The Delray Medical Center patient enrolled in the clinical trial received the first of three treatments at the hospital in February.
In the FDA-approved clinical trial, focused ultrasound technology is used to disrupt the blood-brain barrier in participating Alzheimer’s patients. Alzheimer’s may be caused by a buildup of certain proteins in the brain. Precisely guided by magnetic-resonance imaging, ultrasound waves are directed at specific areas of the patient’s brain to create a temporary opening in the blood-brain barrier where the protein buildup may be reduced. The Delray Medical Center patient enrolled in the clinical trial received the first of three treatments at the hospital in February.
“Delray Medical Center is proud and excited to be a leader in this effort to determine the safety and efficacy of this potentially revolutionary treatment for Alzheimer’s patients,” said Lloyd Zucker MD, FAANS a board-certified neurosurgeon and Medical Director of Neurosurgery at Delray Medical Center. “The study will help determine whether the use of this non-invasive focused ultrasound technology can lead to cognitive improvement in patients with Alzheimer’s disease.”
An estimated 6.5 million Americans ages 65 and older are living with Alzheimer’s disease, which is the sixth leading cause of death for those age 65 and above in the U.S., according to the Alzheimer’s Association. Florida has the second highest incidence of Alzheimer’s in the country, with an estimated 580,000 cases.
The clinical trial, called ExAblate Blood-Brain Barrier (BBB) Disruption for the Treatment of Alzheimer’s Disease, is designed to evaluate the safety and efficacy of Insightec’s ExAblate Model 4000 Type 2.0 System as a tool for disrupting the blood-brain barrier in probable Alzheimer’s patients. The study, part of Florida’s Brain State initiative, is being conducted at up to eight sites in the country. Patients who meet the specific study criteria receive three focused ultrasound treatments, two weeks apart, and will be followed for five years after the final procedure.
The technology has already been shown to be effective in treating patients with Parkinson’s Disease and essential tremor, a neurological disorder that causes involuntary trembling of the head and hands, preventing people with the condition from performing simple tasks, such as tying shoelaces.



